Read my latest thoughts & opinions on contamination control, ultrapure water, as well as periodic reflections on business and life
Opinions are mine alone and I reserve the right to change them at any time
-
Developing a Contamination Control Strategy for Pharmaceutical Waters. Contamination Sources and Design Considerations.
Annex 1 to Volume 4 of the EU Guidelines for Good Manufacturing Practice for Medical Products for Human and Veterinary Use is legally binding for…
-
What are the actual testing requirements for objectionable organisms in pharmaceutical waters?
Is Monitoring Objectionable Organisms in Pharmaceutical Waters for Non-Sterile Drug Manufacturing an FDA Requirement? There are no testing requirements mandated for objectionable organisms found in…
-
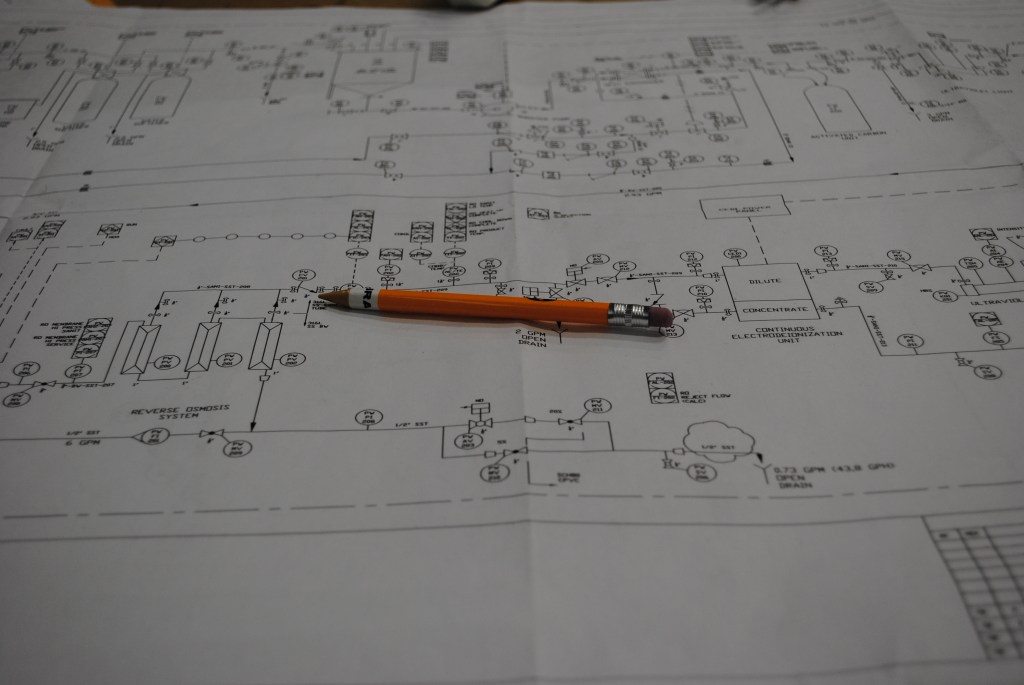
Keys to Design and Monitoring of High Purity Water Systems
A Sound Basis of Design A high purity water treatment system should include technologies designed to remove specific feed water impurities to ensure consistent product…
-
Is Periodic Revalidation of a Pharmaceutical Water System Required?
A risk-based approach to validation includes the flexibility to make continuous improvements to the design, operation, and maintenance of a pharmaceutical water system while still…